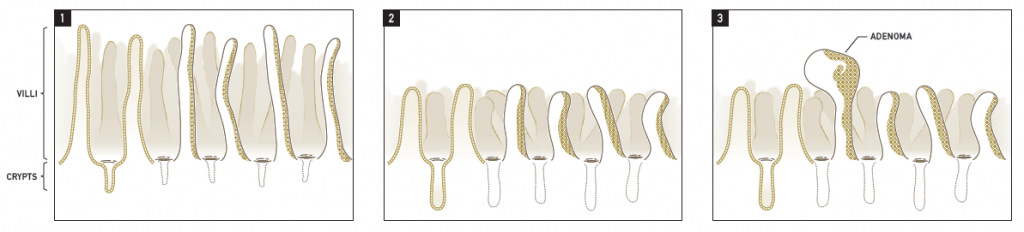
The goal of the Cr(VI) MOA Research study was to inform the Mode of Action (MOA) underlying the oral and intestinal tumors observed in the NTP 2-year study, as well as provide information that regulators could use to inform their risk assessments of Cr(VI). Major study findings are summarized in Thompson et al. (2013), and a brief summary is presented below. However, to understand these findings, one must consider the structure and function of the mucosa lining the small intestine. As shown in Figure 1.1 below, the small intestine is lined by finger-like projections called villi. Enterocytes lining the intestinal villi absorb nutrients from foodstuffs, primarily in the duodenum and proximal jejunum. Nutrients are transported by the villous enterocytes from the lumen and into the bloodstream. These absorptive enterocytes lining the villi are highly proliferative in nature and die and slough into the lumen within several days of cell genesis. These dying villous cells are constantly replenished by epithelial cells that are ‘born’ in the crypt (below the intestinal surface) and migrate to the villus where they eventually slough into the lumen of the intestinal tract, all within a matter of approximately three days. It is generally well-accepted that tumors originate from mutations in the stem cells that reside at the base of the intestinal crypts as opposed to the epithelial cells lining the intestinal villi.
Findings from the Cr(VI) MOA Research Study suggest the sequence of key events depicted in the figure above. Normally, duodenal villi are long and narrow, and the crypts comparatively short (Figure 1.1). Absorption of Cr(VI) at the intestinal villi leads to cytotoxicity within the villous portion of the mucosa, as evidenced by cytoplasmic vacuolization and blunting. This toxicity leads to an increased demand for new epithelial cells to replace the damaged villi, and results in an increase in proliferating epithelial cells within the crypt and a concomitant lengthening of the crypt depth (Figure 1.2). This increased regenerative proliferation and cell division in an already highly proliferative tissue leads to an increase probability of spontaneous mutations, perhaps also potentiated by an oxidative environment due, in part, to infiltration of immune cells. This increase in spontaneous mutation due to intestinal wounding and regeneration over the entire lifespan of the mouse leads to an increase in intestinal tumor (e.g. adenoma) formation (Figure 1.3). Notably, NTP reported that villous blunting and crypt hyperplasia did not occur in rats, and these animals did not develop intestinal tumors after exposure to Cr(VI) in drinking water. In fact, a similar disparity in intestinal cancer between mice and rats has been noted for other intestinal carcinogens, and a similar cytotoxicity-based MOA has been accepted for those compounds by both scientists and regulators (Federal Register, 2004).
The implications of this MOA for Cr(VI) is that intestinal tumors occurred in mice as a result of long-term wounding and healing in response to lifetime exposure to very high concentrations of Cr(VI) in drinking water. Doses where such wounding does not occur are unlikely to pose any carcinogenic risk to rodents or humans. These findings, in conjunction with sophisticated pharmacokinetic models for chromium disposition (Kirman et al., 2012, 2013), can help regulators in risk assessment and determining safe drinking water levels for Cr(VI) that pose no intestinal cancer risk to humans.
References:
Thompson, C.M., Proctor, D.M., Suh, M., Haws, L.C., Kirman, C.R., and Harris, M.A. 2013. Assessment of the mode of action underlying development of rodent small intestinal tumors following oral exposure to hexavalent chromium and relevance to humans. Crit Rev Toxicol., 43(3):244-274.
Kirman, C.R., Hays, S.M., Aylward, L.L., Suh, M., Harris, M.A., Thompson, C.M., Haws, L.C., and Proctor, D.M. 2012. Physiologically based pharmacokinetic model for rats and mice orally exposed to chromium. Chem Biol Interact., 200(1):45-64.
Kirman, C.R., Aylward, L.L., Suh, M., Harris, M.A., Thompson, C.M., Haws, L.C., Proctor, D.M., Lin, S.S., Parker, W., and Hays, S.M. 2013. Physiologically based pharmacokinetic model for humans orally exposed to chromium. Chem Biol Interact., 204(1): 13-27.
Federal Register. 2004 “Captan; Cancer Reclassification; Amendment of Reregistration Eligibility Decision; Notice of Availability,” 69 Federal Register 226 (24 November 2004), pp. 68357-60.